Research
Improvement of photocurrent generation by introduction of conjugated side chain.
In the study of organic solar cells, the development of semiconducting polymers which absorb light in long wavelength regions is a very important research topic in the effort to collect a greater percentage of the solar irradiation. One of the strategies to design these low band gap polymers is to use a combination of electron donating and accepting monomer units to synthesize so called D/A type copolymers. The light absorption accompanied with intrachain charge transfer enable to use lower energy photons (i.e. longer wavelength light) for the excitation. There have been many reports on the synthesis of the D/A type copolymers and it is now possible to design the polymers with a desired absorption spectra. However, it is still not clear how to design the polymers that show very efficient charge transfer successive to the light absorption.

Our team developed a simple, effective approach to modifying D–A type polymers; copolymerization with a conjugated side chain. The resulting copolymers showed significant improvement (25%~43%) in power conversion efficiency in comparison to the original polymers. The short-circuit current of the low band gap polymer reached more than 22 mA cm−2 that is the highest record for organic solar cells to date. This design strategy opens up a new possibility to modify the existing D–A type photovoltaic polymers for better performance.
- polymer, 2013, 54, 6501-6509. DOI
- Energy Environ. Sci., 2012, 5, 9756-9759. DOI
- Macromolecules 2008, 41, 8302. DOI
Polymer blend solar cells
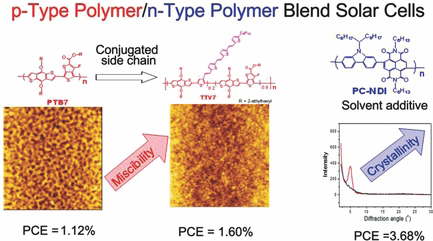
Soluble fullerene derivatives are used in most of the solution-processed organic solar cells, due to the superior electronic properties of the fullerenes. The use of semiconducting polymers as the electron acceptor, however, would improve the flexibility of the molecular design vs fullerene derivatives and could open up a new field of the research to improve the device efficiency.
Our team is working on the material development for the polymer blend solar cells. Control of the structure of two immiscible polymers in the mixed thin films is the key factor to achieve high efficiency. The search for suitable polymer structures for both the donor and the acceptor and the use of solvent additives has led to the better miscibility and crystallinity of the polymers, resulting in an achieved power conversion efficiency of 3.6% for an all-polymer solar cell.
- Adv. Mater. 2013, 25, 6991–6996. DOI
- Chem. Commun., 2012, 48, 5283-5285. DOI
- Angew. Chem. Int. Ed., 2011, 50, 2799-2803. DOI
New polymer design by connecting donor oligomers with acceptor linker
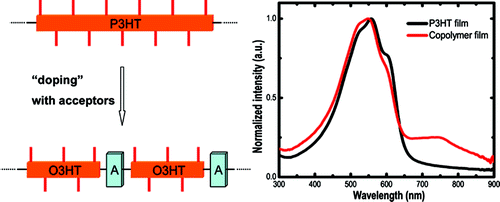
General D/A type approach for the design of low band gap copolymers has the advantage of good control of absorption spectra by changing monomer unit combinations. On the other hand, the crystallinity of the polymers and the charge mobility in thin films is heavily affected by the unit structures. We have established a design to improve the absorption efficiency while maintaining the crystallinity of the polymers. Poly(3-hexylthiophene) (P3HT) is a well-known crystalline semiconducting polymer. In our design, the oligomer of P3HT was connected by electron accepting linkers to produce longer alternating copolymers. In this way, we could expect high crystallinity of the polymer similar to P3HT and simultaneously broader absorption due to the intramolecular interaction close to the acceptor unit. The absorption spectrum in the longer wavelength region can be controlled by changing the length of the oligomer blocks. The results suggest there is intrachain energy transfer from the P3HT oligomer block to the acceptor linker unit.
- Macromolecules, 2011, 44, 4222–4229. DOI
- Macromol. Rapid Commun., 2012, 33, 658-663. DOI
- Polymer J., 2012, 44, 1145–1148. DOI
Nanostructure control by using semiconducting diblock copolymers
The organic solar cells with high performance reported to date rely on so-called bulk heterojunction structures, in which the donor and the acceptor molecules are mixed together in thin films. These structures have large interfacial area between two materials, resulting in the increase of the molecular junction for the charge separation and therefore the increase of the photocurrent. The completely mixed structure is not desirable because efficient transports of holes and electrons should take place in the pure donor and acceptor materials, respectively. Therefore, it is necessary to control the size of the phase separation in bulk heterojunction to achieve both the large interface and the construction of the charge transport pathways. However, one cannot be sure whether this optimized mixed structure could increase the efficiency of the organic solar cell up to its limit or not.
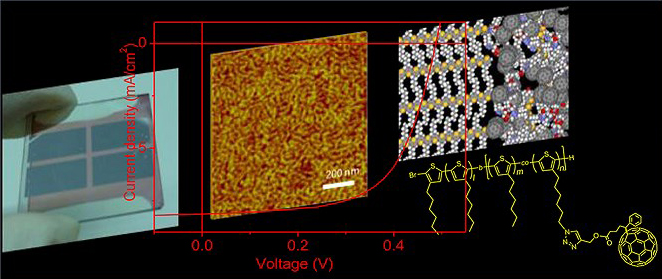
We have been working on the nanostructure control by using self-organization of semiconducting block copolymer, aiming at the construction of “ideal” nanostructure that is superior to the mixed bulk heterojunction. Block copolymers consist of two different polymer blocks connected at the ends, with each block can have a different function. If the two block are immiscible to each other, it is known that the block copolymer can spontaneously form microphase separated structures in the scale of several tens of nanometers. The ability to obtain the block copolymer with the donor and acceptor functions in each block, make them form the microphase separated structure, and use them for the organic solar cells, we can achieve an ideal nanostructure without relying on the mixing of the materials. So far, we successfully synthesized the block copolymer with the donor polymer block and the fullerene-attached acceptor block. The diblock copolymer films formed clear nanostructures with sizes of ca. 20 nm, driven by crystallization of the poly(3-hexylthiophene) (P3HT) block and aggregation of the fullerene groups, as observed in AFM phase images. These results indicate that rational material designs enable the construction of suitable donor−acceptor nanostructures for photovoltaic applications.
- Macromolecules, 2012, 45, 6424−6437. DOI
- Chem. Commun., 2010, 46, 6723-6725. DOI
- J. Am. Chem. Soc. 2008, 130, 7812. DOI
Structure control by using donor/acceptor dyad molecules
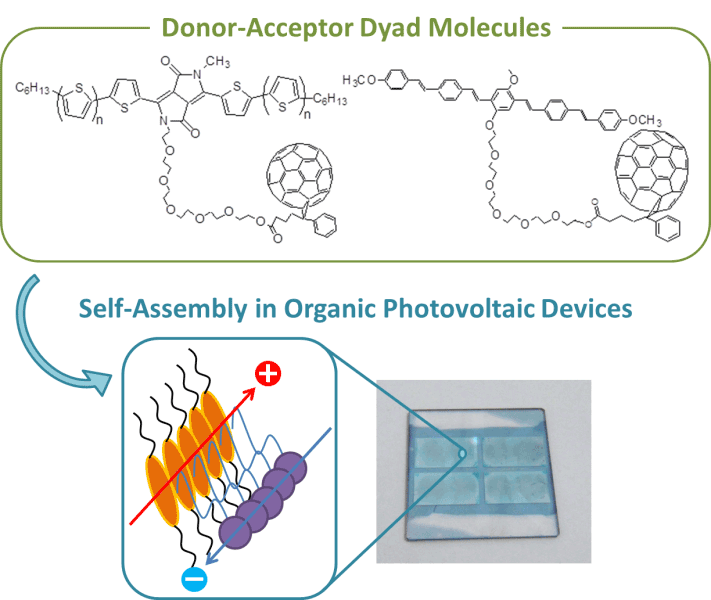
Dyad molecules are defined as a covalently attached donor and acceptor. They have been widely studied with a primary aim of gaining fundamental understanding of charge separation in solution. Application of dyad molecules into organic photovoltaic devices has several advantages over widely used mixed bulk heterojunctions devices in terms of nanostructure control and device reproducibility. Since the donor and acceptor molecules are connected to each other, phase segregation is suppressed, and higher charge separation, owing to the proximity of the donor and the acceptor, leads to current generation. However, the reported power conversion efficiencies of OSCs based on dyads were lower than 0.4 % in early studies. Our group has reported several dyad molecules with highly crystalline π-conjugated oligomers or dyes which have broad absorption in visible region as donors and fullerene derivatives as acceptors. The efficiency has been improved from about 0.1% in early studies to nearly 2% in recent reports. Furthermore, covalent attachment in the dyad prevents large phase separation, resulting in good morphological and device stability at high temperatures as compared with mixed bulk heterojunction devices. These studies demonstrated the considerable promise of the donor–acceptor dyad approach for realizing efficient and stable organic solar cells.
- Chem. Commun., 2009, 2469-2471. DOI
- Chem. Commun., 2011, 47, 6365-6367. DOI
- Phys. Chem. Chem. Phys., 2012, 14, 16138-16142. DOI
Modification of D/A interface with molecular dipole layers
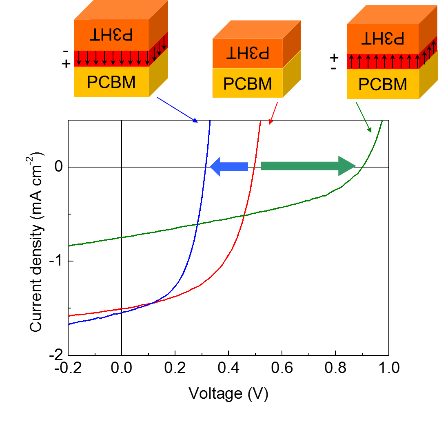
The interfacial properties of organic semiconducting materials are important to ascertain the working mechanisms of organic electronic devices such as organic solar cells, as well as to improve their performance. Organic/organic interfaces formed by wet processes, however, are extremely difficult to fabricate in well-defined manner and to analyze by conventional techniques because they are varied deep inside the films.
We are working on the development of various tools to investigate the relationship between the interfacial structure of the organic materials and the device performance. Using the developed film transfer method and the surface segregated monolayers, we fabricated bilayer organic photovoltaic devices with interfacial dipole moments that were selected to align the energy levels at the heterojunction. As a result, open-circuit voltages of the P3HT/PCBM devices could be tuned over a wide range between 0.3 and 0.95 V, corresponding to the alignment of energy levels at the interface. This observation indicates that even if the same combination of bulk materials is used, the interfacial properties drastically alter the performance of organic photovoltaic devices.
- Phys. Chem. Chem. Phys., 2012, 14, 3713 - 3724. DOI
- Nature Mater., 2011, 10, 450–455. DOI
- Nature Asia Materials Research Highlight
Increase VOC without loss of JSC in organic solar cells
The short circuit current density (JSC) and the open circuit voltage (VOC) are two key factors that have critical influence on the performance of organic solar cells (OSCs). In the course of the material development for OSCs, however, JSC and VOC often show trade-off relationship, especially when approaching high efficiencies. Roughly speaking, this could be attributed to the fact that the efficient D/A interfaces for the charge separation (lead to high JSC) is also efficient for the charge recombination (lead to low VOC). We speculated that the introduction of “charge separation center” at the interface with intrinsically bad for the charge generation/loss process could be a key to construct the asymmetric interface (i.e. good for the charge separation but bad for the charge recombination). By using the model system of bilayer solar cells, we have found that separating the donor and acceptor layer in bilayer OSCs with a thin insulating layer increases the energy of charge transfer (CT) state by weakening the Coulomb interaction at the interface and this also suppresses photoinduced CT and recombination. Although these effects usually increase VOC and decrease JSC, the trade-off is avoided by doping the insulating layer with a dye to utilize the energy transfer process. The increase in VOC without the reduction in JSC enhances the conversion efficiency of the OSCs by 30%.
- Adv. Energy Mater. 2013, in press. DOI
Control of interfacial structures by electric field and change of device performance
Usually we assume that the molecular structures at the organic/organic interfaces in the organic electronic devices are changeless, but if they can be changed by responding to the external stimuli, we can expect that it would change the properties of the devices. Especially for the donor/acceptor interface of organic solar cells at which all the charge generation and the recombination take place, the change of the device could be drastic, therefore it could have some applications such as sensors.
We discovered that the interfacial molecular dipole moment at the D/A interface of the bilayer organic solar cells interact with the applied electric field to change the direction, resulting in the large change of the diode and solar cell properties (namely turn-on voltage and VOC, respectively). These changes are reversible by applying the electric field in the opposite direction. This phenomenon could lead to the development of the devices with new functions such as photodiodes with memory effect.
- Adv. Mater. 2013, 25, 1071-1075. DOI
Vertical orientation of polymer chains in thin films by self-organization
Polymers are in general one-dimensional string-like object that are consist of monomers linearly connected to each other. Semiconducting polymers, however, have π-conjugated plane and often very rigid structure, therefore they are more like a long ribbon-shaped objects. In the polymer thin films, the molecules are oriented in all the directions with some aggregation or crystallized domains, depending on the nature of the intermolecular interactions. Since the charge conduction along the polymer chains (intra-chain process) are often much faster than the other direction (inter-chain process), it is known that when the polymer chains are aligned in one direction in the films, the charge mobility in that direction get much larger than the other directions. It is relatively easy to align the chain in parallel to the substrate or the surface, however, the vertical alignment of the polymer chain in the thin films is challenging and never achieved for whole thin film.
We realized this vertical orientation of the chains by using surface segregation phenomenon, in which the fluoroalkyl chains self-segregate to the liquid/air interfaces due to their low surface energy. The seminconducting polymer with the chain end of the fluoroalkyl chain was synthesized and spin-coated from the solution containing the ordinary semiconducting polymer. During the film formation, the polymer with the fluoalkyl chain was segregated at the surface and vertically oriented. In addition, the ordinary polymer in the bulk of the films also took preferential vertical orientation, probably due to the crystallization from the surface. As the result of this orientation change, the charge transport in the vertical direction showed 30-times enhancement compared to that in the film with parallel orientation. This approach could be also applicable to the other kind of crystalline polymers and it could lead to the improvement of various electronic devices.
Surface segregated monolayer of semiconducting polymers
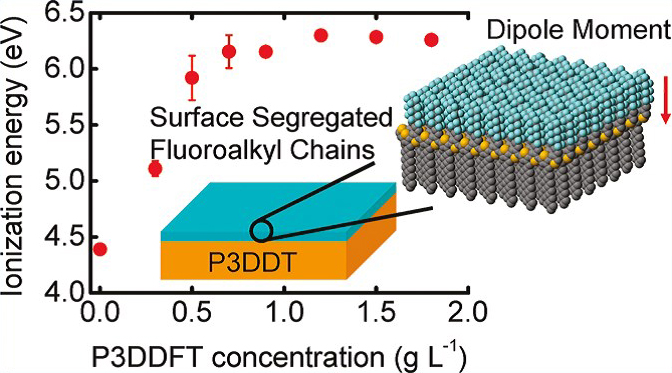
We previously showed that fullerene derivatives with fluoroalkyl chains (FCn) spontaneously form a monolayer on the surface of a nonfluorinated fullerene derivative (PCBM) film during the coating process. We named it surface segregated monolayer (SSM). Aligned fluorocarbon chains form a surface dipole moment that changes the surface energy structure of PCBM films. Extending the concept of SSM to semiconducting polymer films would be of great value in the field of organic electronics.
We synthesized a poly(3-alkylthiophene) derivative with alternating substitution of semifluoroalkyl and alkyl side chains and applied it for SSM on the surface of the semiconducting polymer film. As the result, we found that the semifluoroalkyl chains aligned at the air/solid interface form a large molecular dipole moment that continuously shifts the ionization energy by as much as +1.8 eV depending on the surface density. This can be a novel approach to controlling the energy-level alignment at organic/(in)organic interfaces, analogous to the work function shifts of metals induced by the formation of SAMs. We also found that the alternating sequence of the side chains of the semifluoroalkyl and the alkyl chains is important to form the high density, well-ordered SSM on the film surface.


